Abstract
Introduction CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) remains the standard treatment for older adults with non-anaplastic large cell lymphoma (ALCL), nodal peripheral T-cell lymphomas (PTCLs). However, recent studies suggest that patients (pts) > 75 years (y) have similar outcomes with CHOP compared to those receiving palliative regimens (Ellin et al. Hematological Oncology 2017). In addition, with studies demonstrating sensitivity of PTCLs to epigenetic therapies, especially in the T-follicular helper (TFH) subtypes, there are emerging clinical trials evaluating these agents in newly diagnosed PTCL patients > 60 y (NCT02232516). We evaluated the long-term outcomes of pts aged 65 y or older with PTCLs, a group generally not transplant eligible, treated with systemic therapy with a focus on those treated with curative intent CHOP(like) chemotherapy.
Methods The BC Cancer Lymphoid Cancer Database was reviewed and pts > 65 years with PTCL, not otherwise specified (PTCL-NOS), angioimmunoblastic T-cell lymphoma (AITL) as well as other T-Follicular Helper PTCL (collectively referred to as TFH T-cell lymphoma [TFHTCL]) diagnosed between 2000-2021 and who received front-line chemotherapy were included. Diagnoses were based on the World Health Organization (WHO) classifications of 2001, 2008, and 2017, depending on the era of diagnosis. Time to progression (TTP) was measured from the date of pathologic diagnosis to the date of relapse/progression, death due to treatment toxicity or lymphoma.
Results 152 pts were initially identified, of which 5 were excluded: stage IA/IAE (n=3); CNS involvement only (n=2). Of the remaining 147 pts, 67 (46%) had PTCL-NOS and 80 (54%) had TFHTCL. The median age was 74 y (range 65-90) and the majority of pts had high risk features: International Prognostic Index (IPI) score of > 3 (n=113, 76%), stage III/IV disease (n=136, 93%) and PS of > 2 (n=85, 58%).
Most pts (n=127, 86%) received multi-agent chemotherapy with curative intent. All pts received CHOP/CHOP-like chemotherapy (including five pts treated with CHP-brentuximab vedotin)Over half of these patients (n=66, 52%) received attenuated doses of chemotherapy starting from cycle 1, typically 50-75% dose intensity. Only two patients received consolidative autologous stem cell transplant. The remaining 20 patients received chemotherapy with palliative treatment intent, the majority (n=18, 90%) receiving cyclophosphamide alone or with the CVP (cyclophosphamide, vincristine, and prednisone) regimen.
The median follow-up of all pts using the reverse Kaplan-Meier method was 9.2 y (range 0.9 to 12.5). The estimated 5-y TTP and overall survival (OS) for all patients were 21%, and 26%, respectively. Outcomes were similar in PTCL-NOS and TFHTCL (5-y TTP 17% vs 25%; p = 0.26). Curative intent chemotherapy was used more frequently in the 65-74 y vs > 75 y age group (70% vs 36%, p = 0.004). As expected, outcomes were superior in those treated with curative intent (Table 1): Median TTP of 11.3 months (m) vs 3.0 m; median OS of 24 m vs 4.5 m. Improvements in TTP and OS extended to those > 75 y (p < 0.001 for both).
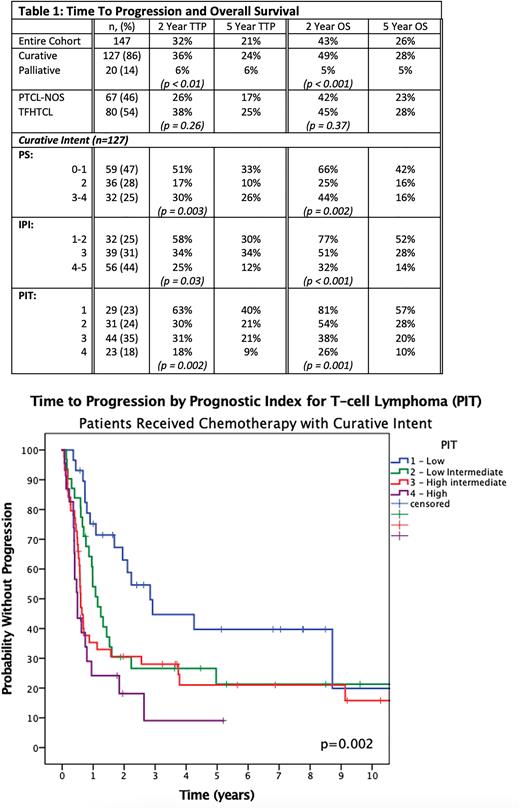
Considering the curative intent cohort, 91% of the relapse/progression events occurred within the first 3 y, and there was no significant difference in outcomes (5-y TTP/OS) with increasing age: 65-75 (19%/25%), >75 (34%/35%) (p = 0.08/0.6). Further, the outcomes of pts who received attenuated doses of CHOP-like chemotherapy were not inferior compared to full dose chemotherapy (5-y TTP/OS 32%/37% vs. 17%/21% p= 0.2/0.3). 5-y TTP/OS by the IPI ranged from 30%/52% for IPI of 1-2 to 12%/14% for IPI 4-5. The prognostic index for T-cell lymphoma (PIT) was more effective at identifying a lower risk group: 5-y TTP/ OS (PIT 1 40%/57%; PIT 2 21%/28%; PIT 3 21%/20% PIT 4 9%/10%, p = 0.002/0.001). Both models were prognostic in PTCL-NOS and TFHTCL. (Table 1)
Conclusion With mature follow-up, ~ 30% of all older pts treated with curative intent CHOP-like chemotherapy remain progression-free and are alive at 5 y. Although results remain suboptimal, robust older patients can still be considered for curative intent CHOP chemotherapy with tailored doses to mitigate toxicity. Those with low risk IPI/PIT scores have a more favourable outcome with this approach, with a 5-y OS of > 50%, suggesting a benefit of subsequent therapies. In contrast, higher risk disease have poor outcomes and novel therapeutic approaches should be considered.
Disclosures
Ngu:AstraZeneca: Honoraria. Villa:AstraZeneca, Roche: Research Funding; Roche, AstraZeneca, Abbvie, Janssen, Kite/Gilead, BMS/Celgene, BeiGene, Kyowa Kirin: Consultancy, Honoraria. Gerrie:AstraZeneca: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Sandoz: Honoraria; Janssen: Honoraria, Research Funding. Venner:Janssen: Honoraria; FORUS Therapeutics: Honoraria; Takeda: Honoraria; BMS: Honoraria; GSK: Honoraria; Sanofi: Honoraria; Pfizer: Honoraria. Craig:BeiGene: Honoraria; Bayer: Consultancy. Slack:Seagen: Honoraria. Scott:Incyte: Consultancy; AstraZeneca: Consultancy, Honoraria; NanoString: Patents & Royalties; Janssen: Consultancy, Research Funding; Roche: Research Funding; Abbvie: Consultancy. Sehn:AbbVie, Acerta, Amgen, Apobiologix, AstraZeneca, BMS/Celgene, Debiopharm, Genmab, Gilead, Incyte, Janssen, Kite, Karyopharm, Lundbeck, Merck, Morphosys, Novartis, Sandoz, Seattle Genetics, Servier, Takeda, TG Therapeutics, Verastem: Consultancy; Chugai: Consultancy, Honoraria; AbbVie, Acerta, Amgen, Apobiologix, AstraZeneca, BMS/Celgene, Gilead, Incyte, Janssen, Kite, Karyopharm, Lundbeck, Merck, Morphosys, Sandoz, Seattle Genetics, Servier, Takeda, TG Therapeutics, Verastem: Honoraria; Teva, Roche/Genentech: Consultancy, Honoraria, Research Funding. Savage:BMS, Janssen, Kyowa, Merck, Novartis, and Seattle Genetics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Beigene and Regeneron: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal